
Daniell or Galvanic Cell:
Section 19.2, pg. 763, Figure. 19.1, Burge.
In its most primitive form a Galvanic cell can be produced when two dissimilar metals are placed together separated by a moistened piece of paper soaked in salt water.

The above figure shows copper and zinc disks piled together and the electrolyte as a piece of paper soaked in a salt solution to act as an electrolyte.
One type of Galvanic cell produces an electric current with an oxidation-reduction reaction between metallic zinc and copper ions in the form copper sulfate. Other combinations can be used by consulting the activity series.
In the figure below, a typical experimental apparatus is shown.
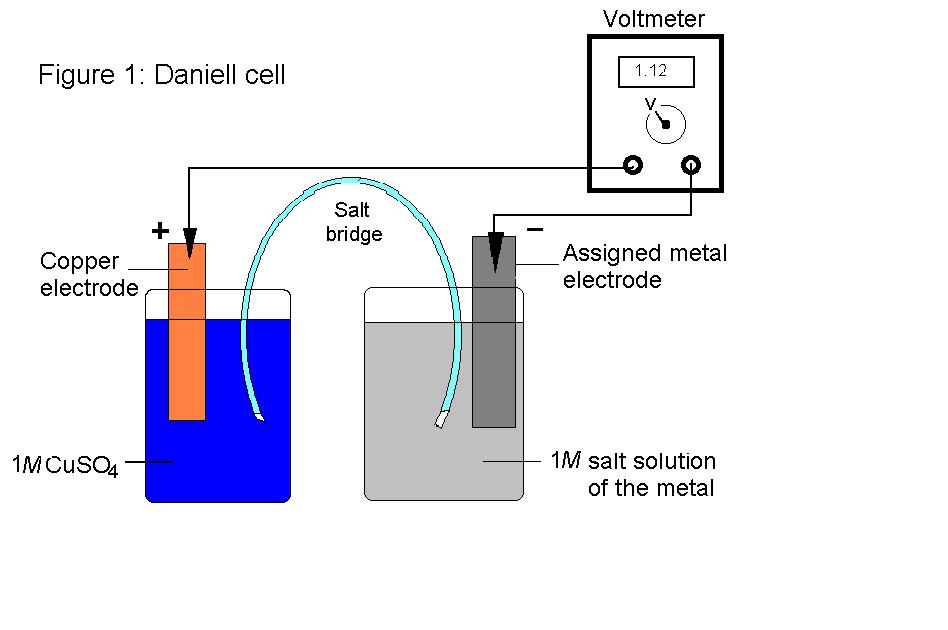 .
.
The apparatus shown above consists of a strip of copper in a copper sulfate solution, a strip of zinc in a zinc sulfate solution and a salt bridge between them that allows ions to pass, but does not permit the solution to mix. The two ends of the bridge are sealed by some porous material like packed cotton. The two metals are connected by a wire which in this case has a voltmeter in series.
The copper strip acts as a cathode. This is where copper ions are abstracted from the solution and deposited in the form of copper metal. This is where reduction occurs.
The zinc strip acts as an anode. This is where zinc metal is converted to a zinc cation in the form of zinc sulfate with the loss of electrons. This is where oxidation occurs.
When zinc loses two electrons, they are passed through the wire and used for the reduction of copper ions to make copper metal.
For every copper ion lost two positive sodium cations diffuse through the salt bridge to balance the charge. For every zinc cation added to the solution of zinc sulfate another anion of sulfate migrates into the solution of zinc sulfate.
This serves to keep the over all charge balance of the entire cell electrically neutral.